Alpha-particle Scattering and Rutherford's Nuclear Model of Atom
Alpha-particle Scattering and Rutherford's Nuclear Model of Atom: Overview
This topic consists of various concepts like Rutherford Experiment,Scattering Angle in Rutherford Experiment,Interpretation of Rutherford Experimental Results, etc.
Important Questions on Alpha-particle Scattering and Rutherford's Nuclear Model of Atom
Experimentally it is found that energy is required to separate a hydrogen atom into a proton and an electron. So the orbital radius of the electron in a hydrogen atom is .
The value of the is: _____.
( and electronic charge )
An electron in a hydrogen atom makes transition from shell to shell . The ratio of initial to final value of magnitudes of centripetal acceleration of electron is
Mention the conclusion of the Geiger-Marsden -particle scattering experiment.
What is the impact parameter in a scattering experiment?
In Rutherford experiment what is impact parameter?
What is impact parameter in Rutherford experiment?
In Rutherford's experiment, a thin gold foil was bombarded by alpha particles. If Thomson's "Plum Pudding Model" of the atom were correct, what would have been the outcome of the experiment?
In the ground state in electrons are in stable equilibrium while in electrons always experience a net force. Here, and refer to
In Rutherford scattering experiment, the correct angle of scattering of α− particles for impact parameter equal to zero is
In Rutherford's experiment, the alpha particles that come closer to the nuclei are
Out of the following statements regarding Rutherford's model, which of the following is/are correct?
a. Most of the space inside an atom is empty.
b. The electrons revolve around the nucleus under the influence of coulomb force acting on them.
c. Most part of the mass of the atom and its positive charge are concentrated at its centre.
d. The stability of atom was established by the model.
At what speed must have electron revolve around the nucleus of hydrogen atom so that it may not be pulled into the nucleus by electrostatic force of attraction
Velocity of electron orbiting around nucleus of hydrogen atom is proportional to radius (r)
Radius of the smallest orbit of hydrogen atom is
Assertion (A): The impact parameter for scattering of - particles by is zero.
Reason (R): Zero impact parameter means that the -particles tend to hit the center of the nucleus.
The total energy of an electron in the first excited state of the hydrogen atom is about . The potential energy of the electron in this state is (in )
Explain briefly how the Rutherford atomic model is not able to explain stability of the atom.
Geiger-Marsden experiment is related with the size of the-
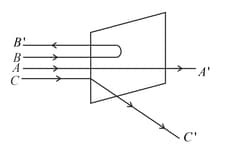
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
The angular momentum of an electron in hydrogen atom is Here is the Plank's constant. The kinetic energy of this electron is
